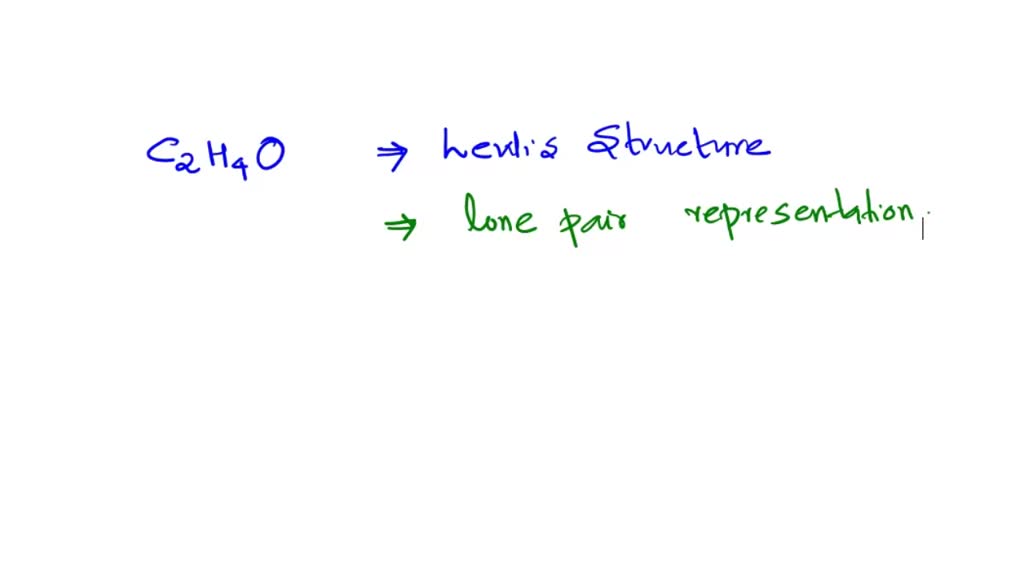
SOLVED Draw the Lewis structure for acetaldehyde (C2H4O). Acetaldehyde
Drawing the Lewis structure for C 2 H 4 (named ethene) requires the use of a double bond. In a double bond two pairs of valence electrons are shared (for a total of four valence electrons). Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. Video.

C2H4O Lewis Structure
Here's how you can easily draw the C 2 H 2 O Lewis structure step by step: #1 Draw a rough skeleton structure #2 Mention lone pairs on the atoms #3 If needed, mention formal charges on the atoms #4 Minimize formal charges by converting lone pairs of the atoms, and try to get a stable Lewis structure

Acetaldehyde Formula C2H4O Explained, Uses, Structures, Examples
Give the Lewis structure for C 2 H 4 O. This formula has at least two possible structures (isomers). Include those structures. Isomers: Compounds having an identical chemical formula but.

C2h4 Lewis Dot Structure
Not to be confused with Ethyl oxide. Ethylene oxide is an organic compound with the formula C2H4O. It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor.

Acetaldehyde Formula Uses, Structures, Examples Embibe
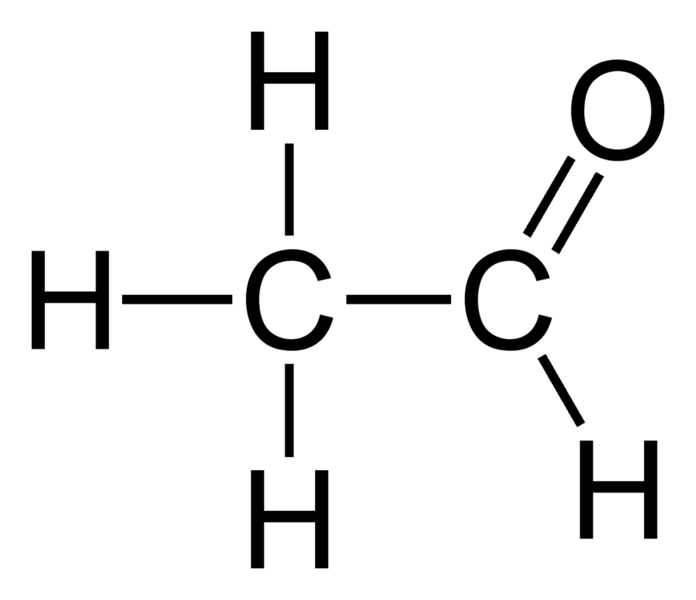
Geometry of Molecules Hi Guys! C2H4O is a chemical formula for ethylene oxide. It is also known as vinyl alcohol, and to find out its Lewis Structure, we first look at the total n.
Quimica Organica Etanal
Draw the Lewis structure for C2H4O2 OneClass 14K subscribers Subscribe Subscribed 4.1K views 2 years ago 🚀To book a personalized 1-on-1 tutoring session: 👉Janine The Tutor.

Acetaldehyde Formula C2H4O Explained, Uses, Structures, Examples
C2H4 Lewis Structure Hydrogen is the least electronegative element here. However, in Hydrocarbons, we always place the Carbon atoms in the center as shown in the figure. We have 12 available valence electrons. Start by forming covalent bonds between the Carbon and Hydrogen atoms.
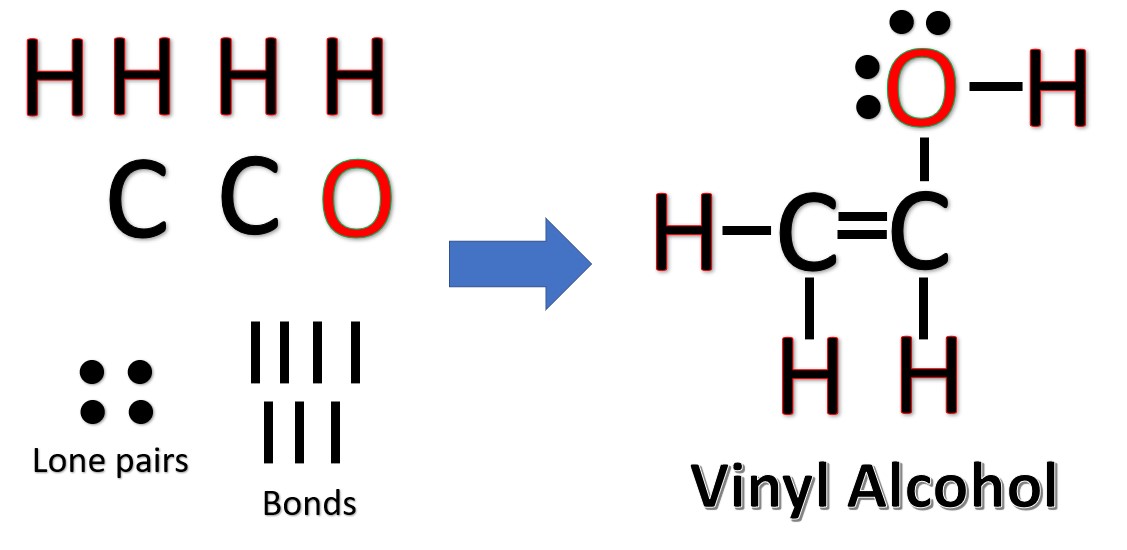
The Lewis Structures of C2H4O [with free study guide and video]
Watch on 6 Steps to Draw the Lewis Structure of C2H4 Step #1: Calculate the total number of valence electrons Here, the given molecule is C2H4 (or ethene). In order to draw the lewis structure of C2H4, first of all you have to find the total number of valence electrons present in the C2H4 molecule.

Locating transition states C2H4 + HCl
Ethylene Oxide | C2H4O | CID 6354 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
CH4O Lewis Structure How to Draw the Lewis Structure for CH4O YouTube
What are the Lewis Structures of C 2 H 4 O? Actually, there are several plausible structures. This molecular formula lead to either acetaldehyde, Ethylene Oxide, or Vinyl Alcohol. All are correct answers. Below, we show two methods to arrive at any one of these answers. What are these molecules?
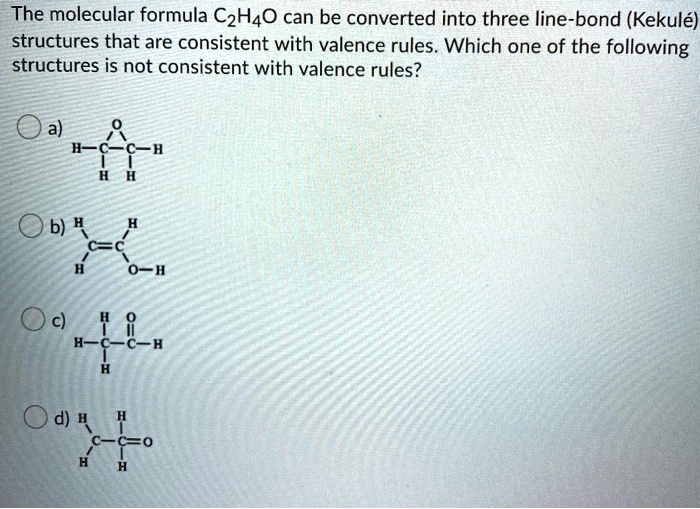
SOLVED The molecular formula C2H4O can be converted into three line
What are the Lewis Structures of C 2 H 4 O? Actually, there are several plausible structures. This molecular formula lead to either acetaldehyde, Ethylene Oxide, or Vinyl Alcohol. All are correct answers. Below, we show two methods to arrive at any one of these answers. What are these molecules?

C2H4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram
C2H4 Lewis Structure The electron dot structure, widely known as Lewis Structure, is a skeletal diagrammatic representation of a molecule taking into account the constituent atoms and the valence shell electrons.

C2H4O Lewis structure, Isomers, molecular geometry, hybridization
The Lewis structure of acetaldehyde (C2H4O) is made up of 2 carbon (C) atoms present at the center while 4 hydrogens (H) and 1 oxygen (O) atom occupy terminal positions. According to the central C-atom, there are a total of 3 electron density regions around the central atom and it has no lone pair of electrons.
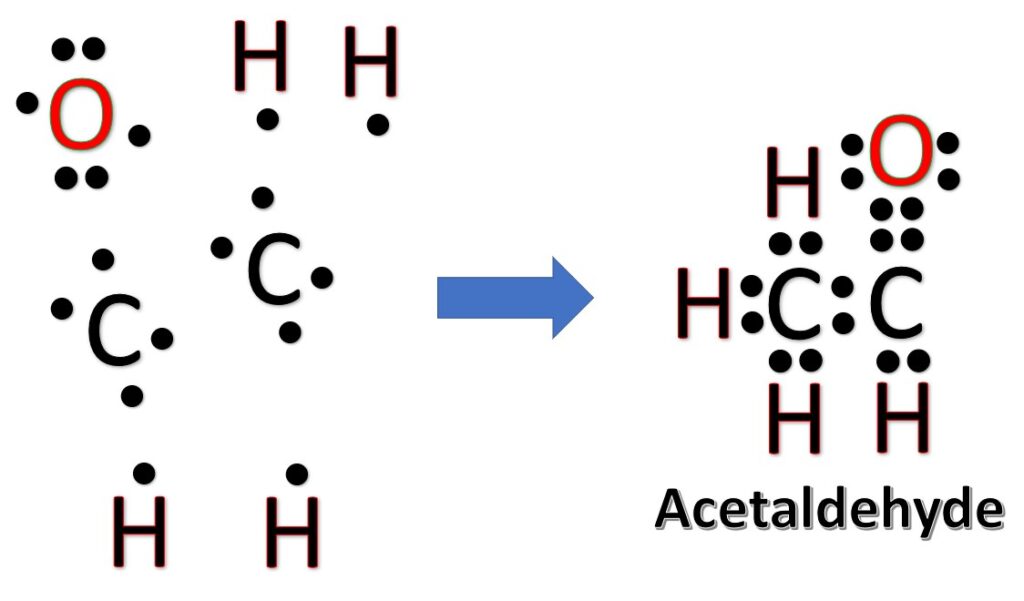
The Lewis Structures of C2H4O [with free study guide and video]
C2H4O2 Lewis structure: How to Draw the Lewis Structure for C2H4O2 Geometry of Molecules 3.12K subscribers Subscribe Subscribed 1 2 3 4 5 6 7 8 9 0 1 2 3 4 5 6 7 8 9 0 1 2 3 4 5 6 7 8 9 1 2.
lewis structure for c2h4o
-111 °C OU Chemical Safety Data (No longer updated) More details-111 °C Jean-Claude Bradley Open Melting Point Dataset 16045-111.7 °C (Literature) Jean-Claude Bradley Open Melting Point Dataset 21302-111.7 °C FooDB FDB003358-172--170 °F (-113.3333--112.2222 °C) Wikidata Q407473-171 °F (-112.7778 °C) Wikidata Q407473-111 °C Sigma-Aldrich SIAL-03906
lewis structure for c2h4o
The information on this page is fact-checked. C 2 H 4 Lewis structure. C 2 H 4 (ethylene or ethene) has two carbon atoms and four hydrogen atoms. In the C 2 H 4 Lewis structure, there is a double bond between the two carbon atoms, and each carbon is attached with one hydrogen atom, and none of the atoms has a lone pair.